Engineering Plastics Business
Making All Key Monomers Sustainable
Polyplastics has previously unveiled LAPEROS® LCP, a plastic made using biomass-derived key monomers. This time, we introduce another important key monomer of LCP as we have begun to study the use of sustainable materials.
Until now, naphthalene-derived monomers have been used quite extensively for grades of LCP used primarily for narrow-pitch connectors. Naphthalene is contained in coal tar, which is generated during the high-temperature process of producing coke from coal. However, the environmental impact is high since carbon dioxide is generated whenever coal is burned. Making such monomers sustainable is thus a key challenge. In order to make all raw materials of LCP sustainable, it is vital that we determine the feasibility of making naphthalene-derived monomers sustainable.
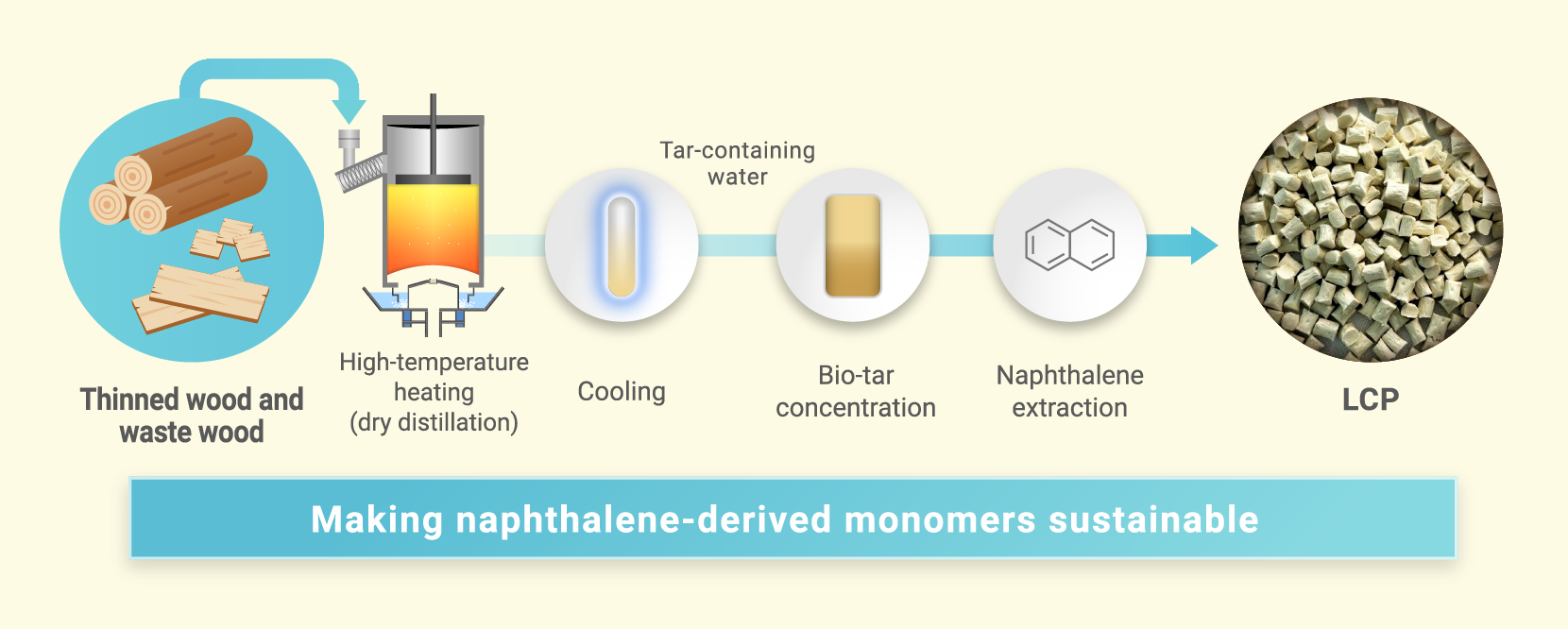

To address this, we turned our attention to a traditional Japanese practice that has been passed down for generations: charcoal making. Charcoal is produced by heating wood at high temperatures in a charcoal kiln through a process known as dry distillation. During this process, gas components are released and can be cooled to obtain wood tar—also known as bio-tar. This method allows for the reuse of resources such as thinned wood and scrap timber, making it an environmentally-friendly approach. Moreover, naphthalene can be extracted from this bio-tar, offering a valuable raw material. In many ways, this traditional technique has emerged as a truly sustainable and promising solution for the future.
Exploring the Potential of Pulp Waste Liquids
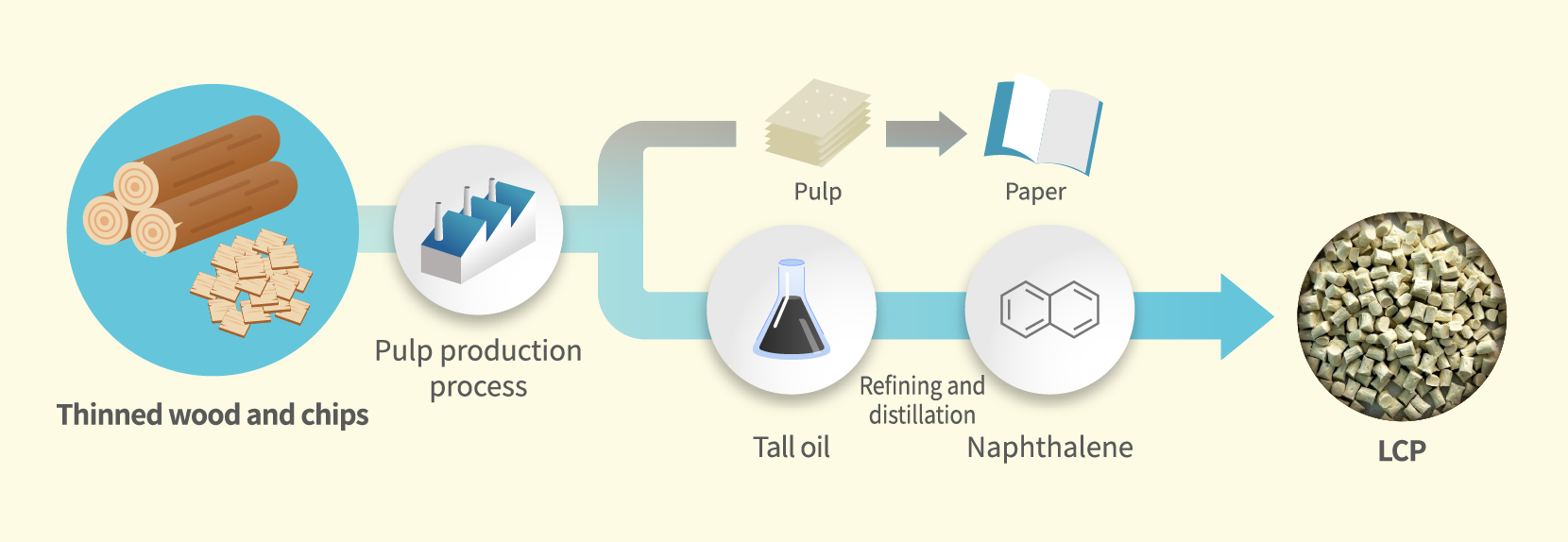

In addition to bio-tar, a method for extracting naphthalene from the liquid waste generated through the process of producing pulp is also being studied. Not only does this approach offer the potential to secure a greater quantity of naphthalene compared to bio-tar, but it also enables the effective use of resources that would otherwise be discarded. This makes it a highly suitable option making it environmentally viable.
LCP grades developed with sustainable raw materials are also superior in terms of thin-walled and high liquidity and are used in narrow-pitch connectors. These narrow-pitch connectors are essential in various mobile devices, wearable terminals, in-vehicle inverters, convertors, battery-monitoring systems, and probe cards for inspecting semiconductors and printed circuit boards and will play an important part in the development of these industrial sectors. The grade of LCP that supports such materials is capable of meeting the needs of our customers' environments, while also contributing to the continued advancement of future society.
We will provide comprehensive environmental solutions as a leading company in the area of engineering plastics while we continue to promote the development of environmentally-friendly products.
New Fermentation Technologies for Obtaining Naphtha
Daicel is known for its advanced fermentation technology. A study on using specific bacteria with sugar as a raw material in a process of fermentation with a low environmental impact to create LCP monomers was commenced by Daicel jointly with our company. Marshalling the capabilities of the Daicel Group will enable LCP monomers to be made even more sustainable.
Related Link
Development of New DURACON® POM Short-Fiber Cellulose Reinforced Grades
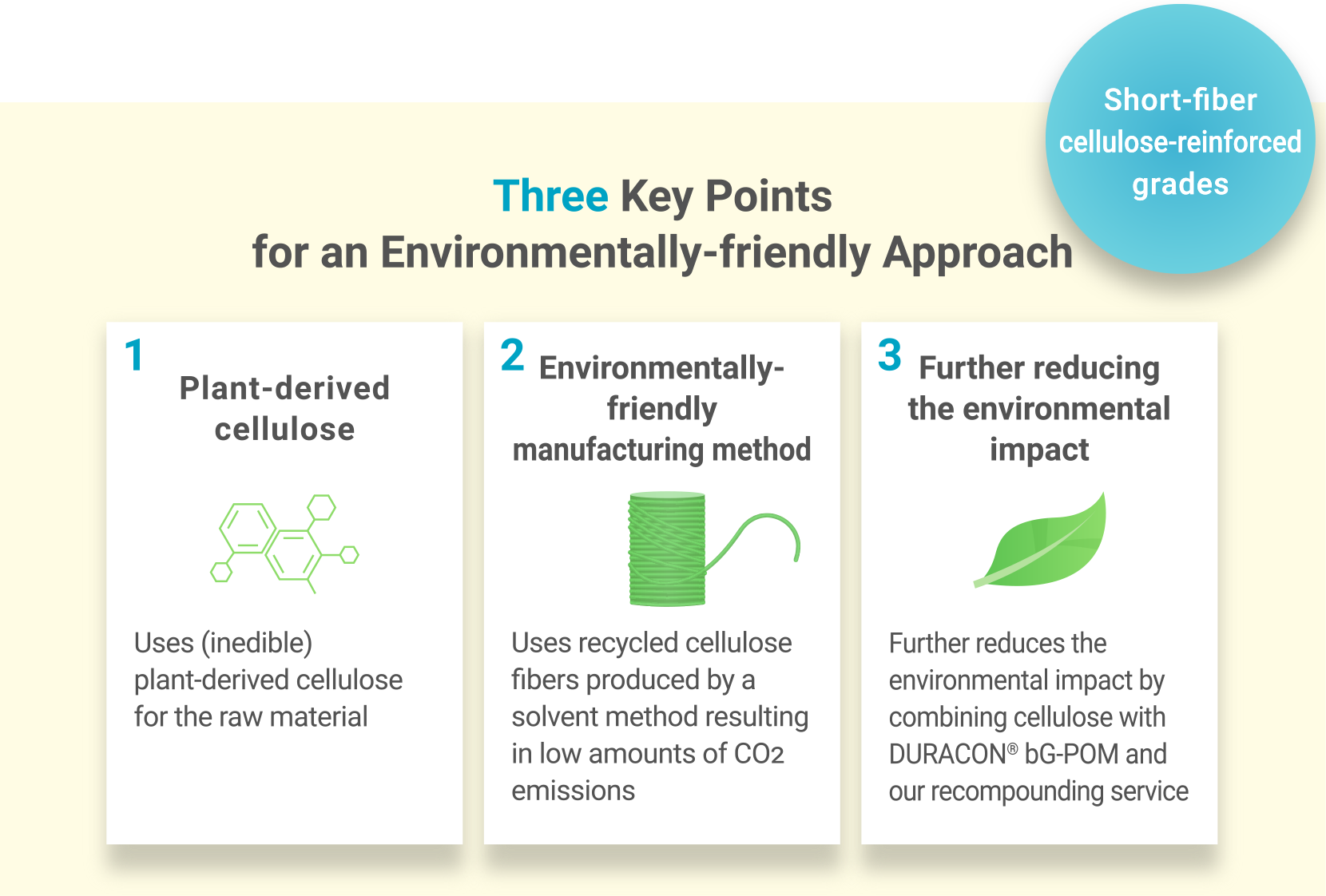
Low-impact materials are gaining attention in building a recycling-oriented society, with plant-derived cellulose offering strong potential. We are developing DURACON® POM grades with short-fiber cellulose to meet environmental needs.
Cellulose is a recyclable, abundant fiber that helps reduce CO2 emissions throughout a product’s life cycle. These grades use recycled cellulose made via a low-waste solvent method, and the fibers come from non-edible parts, improving resource efficiency. Further impact reduction is possible by combining cellulose with DURACON® bG-POM and our recompounding service.
A key feature of this material is its ability to retain POM’s excellent sliding properties. Unlike glass fiber-reinforced POM, which can cause wear on counterpart materials, cellulose enables both high rigidity and smooth sliding. With increased cellulose content, stiffness comparable to glass fiber grades can be achieved.
This grade is suitable for a wide range of applications, including automotive safety parts, mechanical components, daily items, and PC keyboard parts. Its versatility and performance make it a promising solution for sustainable innovation in engineering plastics.

One of the key features of this material lies in its ability to maintain the excellent sliding properties inherent to POM. When compared to glass fiber-reinforced POM grades, which tend to cause wear on counterpart materials during sliding, it has traditionally been difficult to achieve both high mechanical strength and favorable sliding characteristics. However, cellulose fibers offer a unique advantage: they enable the coexistence of superior sliding performance and high rigidity. By increasing the cellulose content, it is even possible to attain an elastic modulus comparable to that of glass fiber-reinforced POM.
This grade is expected to be used in a wide range of applications, similar to conventional POM. These include safety components such as automotive door locks, door clutches, and seatbelt locking mechanisms; mechanical elements like gears, screws, and bearings; everyday items such as fasteners and toothbrush handles; and even parts for PC keyboards. Its versatility and performance make it a promising material for both current and future needs.
We will continue to provide groundbreaking engineering plastic solutions that are both functional and environmentally-friendly.
Realizing Mono-materialization with the Development of Aluminum-Free PTP Sheets
In recent years, as the demand for recycling has rapidly increased in pursuit of a circular society, mono-material products have been gaining significant attention. We have also developed PTP sheets using TOPAS® COC that are free of aluminum as a product that contributes to mono-materialization.
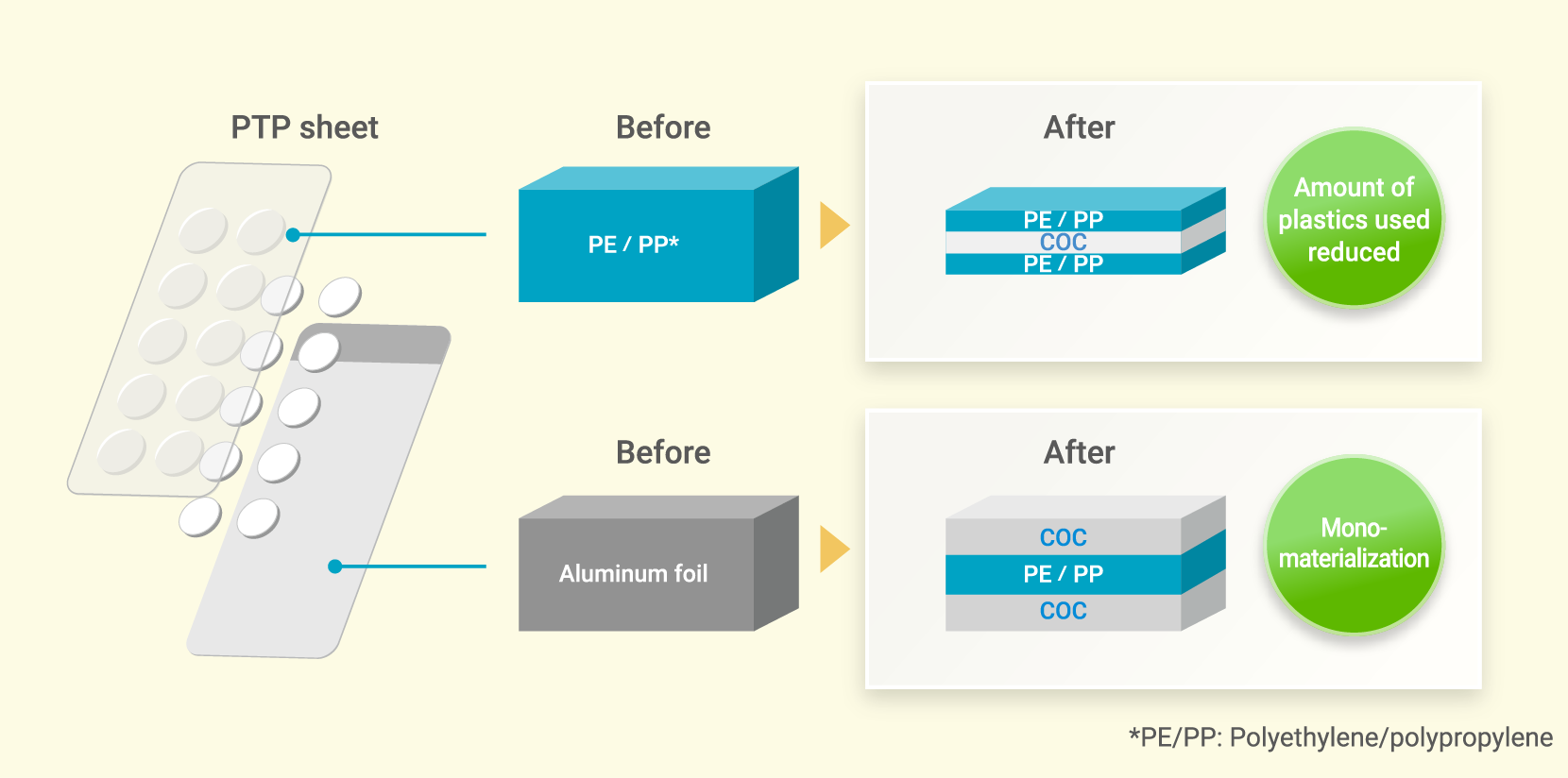
Conventional PTP sheets are commonly used for packaging tablets, capsules, and other medications using two types of materials: plastic and aluminum foil. When recycling these sheets, however, it is difficult to separate these materials from each other with the result that they tend to be discarded rather than recycled, thereby giving rise to a significant environmental impact. To address this challenge, we developed an aluminum-free PTP sheet made using COC.
Possessing excellent chemical resistance and safety properties and thus useful in the medical, pharmaceutical, and packaging fields, COC has excellent affinity with olefin resins, making it possible to dispose of this material together with polyethylene and polypropylene, which have conventionally been used to produce PTP sheets. Since COC has a property that makes film brittle when it is compounded with olefin resins, it can be used as an alternative film to the aluminum foil that is normally used in PTP sheets and recycled as a mono-material. This shift toward mono-material design helps minimize the contamination of different materials during the recycling process, thereby contributing to improved quality.
Compounding COC with olefin resins will lead to improved water vapor barrier properties and enable thinner walls. This in turn will lead to the reduced use of plastics by volume.

Aluminum-free PTP sheets contribute significantly to the realization of a recycling-oriented society and help improve product functionality. With other examples of mono-material packaging, adding and compounding COC can improve polyethylene and polypropylene in such functional terms as their rigidity and barrier properties. Accordingly, we are beginning to see them being applied to all sorts of packaging options. We will continue to work on developing high-value-added products that are both functional and environmentally-friendly.
Related Link
PLASTRON® LFT Cellulose Fiber-Reinforced Grade
The transportation company Asakawagumi Unyu provides a comprehensive array of logistics services, including ground transportation, warehousing, and packaging, on top of its port transport operations. Our PLASTRON® LFT cellulose fiber-reinforced grade has been selected for use in Asakawagumi Unyu’s EMUNEJI.
The EMUNEJI are a plastic packaging material used to fasten reinforced cardboard for transport. While metal fasteners are limited in terms of the tools used to attach them and may require fairly skilled work, EMUNEJI can be easily attached with an electric screwdriver or other such tool, thereby increasing work efficiency. Since the tips of these screws do not protrude, they also enhance safety during handling and transport whenever work is being performed, or goods are being transported. In recent years, there has been a growing demand for packaging materials that contribute to reducing environmental impact.
In response to this trend, our LFT cellulose fiber-reinforced grade—offering both sufficient mechanical strength and the benefits of a biomass-based material—has been selected for use.
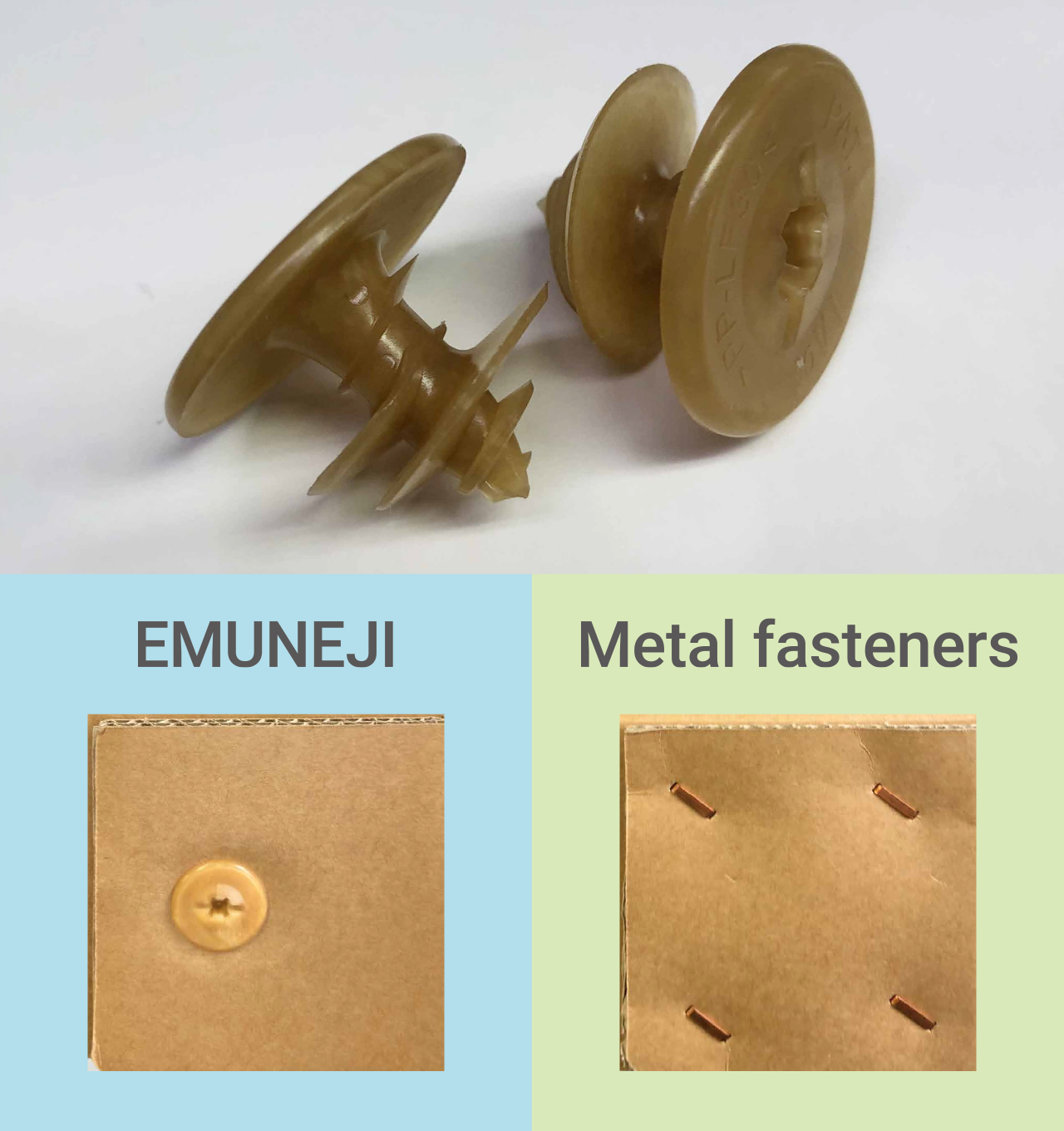
Packaging materials for transport are sometimes used for exporting goods and must, therefore, be tough enough to withstand exposure to harsh environments for logistics. The cellulose fibers used in our newly adopted grade are long fibers, which enable the material to achieve higher impact strength and greater rigidity.
From an environmental standpoint, this cellulose fiber is a plant-derived sustainable material and does not generate residual substances when incinerated, unlike glass fibers and metals, which means that it can undergo thermal recycling, thus helping to reduce industrial waste. Since the amount of energy consumed in manufacturing this cellulose fiber is less than it is for the nylon resin that is used in the general grade of EMUNEJI, the product carbon footprint is also low. Since this grade is lightweight, its use contributes as well to a reduction in carbon dioxide during transportation by reducing emissions associated with transportation materials.
We are actively advancing the development of environmentally-friendly products, including exploring grades that utilize post-consumer recycled (PCR) materials as the base resin for LFT.
Comment from Asakawagumi Unyu
We have been working for several years on incorporating biomass materials into EMUNEJI. Generally, product strength goes down as we use more biomass material. By using the PLASTRON® LFT cellulose fiber-reinforced grade, however, we managed to develop an environmentally-friendly product whose strength is maintained. We definitely look forward to using grades made with PCR materials produced by Polyplastics in the future.

